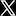iBoost alternatives?
I'm interested in your unused iboost.
We got a givenergy inverter and battery will be fitted soon.
We have Bosch 8000 and an electric immersion.
Regards Jez
Contact me via email.
Jez_dean@hotmail.co.uk
@derek-m, we've switched to an Immersun this week – it's a terrific piece of kit. Well designed. Easy to install. And works ridiculously well.
Get a copy of The Ultimate Guide to Heat Pumps
Subscribe and follow our YouTube channel!
@derek-m, how can I calculate how much water I’m heating per degree centigrade based on diverting 1kWh of solar generation?
Put another way, if I have a 300 litre water cylinder, how much power would I need to divert to raise the temperature by 1C?
Get a copy of The Ultimate Guide to Heat Pumps
Subscribe and follow our YouTube channel!
@editor calculator number 2 says 350w
https://bloglocation.com/art/water-heating-calculator-for-time-energy-power
Posted by: @editor@derek-m, how can I calculate how much water I’m heating per degree centigrade based on diverting 1kWh of solar generation?
Put another way, if I have a 300 litre water cylinder, how much power would I need to divert to raise the temperature by 1C?
It takes approximately 1.16W to heat 1 litre of water by 1C, so 300 litres will take 348W.
We’re reviewing the Immersun, and I came across this formula that I used to calculate the amount of energy required to heat 300 liters of water from 25°C to 44°C:
Q = m * c * ΔT
- Q is the amount of heat energy required, measured in joules (J)
- m is the mass of water being heated, measured in kilograms (kg)
- c is the specific heat capacity of water, which is 4.184 J/g°C
- ΔT is the change in temperature, measured in Celsius (°C) - our old heat pump friend.
First, we convert the volume of water from liters to kilograms, by multiplying it by the density of water, which is approximately 1 kg/liter:
m = 300 kg
Then, we can calculate the change in temperature:
ΔT = (44°C - 25°C) = 19°C
Now, we substitute these values into the formula:
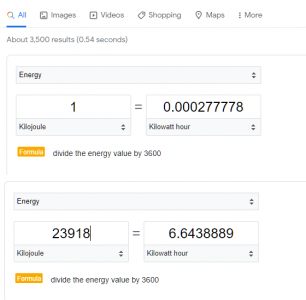
Q = m * c * ΔT Q = 300 kg * 4.184 J/g°C * 19°C Q = 23,918.4 kJ
To convert kilojoules (kJ) to kWh, we divide by 3,600 (the number of seconds in an hour) and multiply by the conversion factor of 0.2778:
23,918.4 kJ ÷ 3,600 s/hour x 0.2778 kWh/kJ ≈ 1.98 kWh
So it would take approximately 1.98 kWh of electricity to heat 300 liters of water from 25°C to 44°C.
Sound right?
Get a copy of The Ultimate Guide to Heat Pumps
Subscribe and follow our YouTube channel!
Posted by: @editorWe’re reviewing the Immersun, and I came across this formula that I used to calculate the amount of energy required to heat 300 liters of water from 25°C to 44°C:
Q = m * c * ΔT
- Q is the amount of heat energy required, measured in joules (J)
- m is the mass of water being heated, measured in kilograms (kg)
- c is the specific heat capacity of water, which is 4.184 J/g°C
- ΔT is the change in temperature, measured in Celsius (°C) - our old heat pump friend.
First, we convert the volume of water from liters to kilograms, by multiplying it by the density of water, which is approximately 1 kg/liter:
m = 300 kg
Then, we can calculate the change in temperature:
ΔT = (44°C - 25°C) = 19°C
Now, we substitute these values into the formula:
Q = m * c * ΔT Q = 300 kg * 4.184 J/g°C * 19°C Q = 23,918.4 kJ
To convert kilojoules (kJ) to kWh, we divide by 3,600 (the number of seconds in an hour) and multiply by the conversion factor of 0.2778:
23,918.4 kJ ÷ 3,600 s/hour x 0.2778 kWh/kJ ≈ 1.98 kWh
So it would take approximately 1.98 kWh of electricity to heat 300 liters of water from 25°C to 44°C.
Sound right?
I get the electrical energy required to be approximately 6.6 kWh.
Where does the x 0.2778 come from?
I also get 23848.8 kJ rather than 23918.4 kJ
Our Immersun PowerDivert has now been running for a month, and we're incredibly impressed with the quality of this British made power diverter. Watch our review here:
Spoiler alert: this product is far superior to the Solar iBoost.
Get a copy of The Ultimate Guide to Heat Pumps
Subscribe and follow our YouTube channel!
@editor I watched the video - looks a great piece of kit. We have the same size PV array as you (6.1kWp) I think. Our average daily solar sent to grid in the winter months (Nov to Feb) is less than 4kWh so it might not be that useful for us in winter (on average, obviously there will be sunny days when it is) but the rest of the year when we are on average sending 8kWh a day to the grid I think it will be a great addition. Question I had from the video - sorry if I missed the information - how many kW is the immersion element in your main tank, and what is the volume? I will be limited to a 2kW immersion (I don't have one as yet but for my Daikin kit, its 2kW) in a 500L tank (thermal store). Thanks for the review!
- 27 Forums
- 2,495 Topics
- 57.8 K Posts
- 292 Online
- 6,220 Members
Join Us!
Worth Watching
Latest Posts
-
RE: Humidity, or lack thereof... is my heat pump making rooms drier?
@majordennisbloodnok I’m glad I posted this. There see...
By AndrewJ , 10 minutes ago
-
RE: Electricity price predictions
i only know about Intelligent Octopus Go ToU, where acc...
By SKD , 50 minutes ago
-
RE: What determines the SOC of a battery?
@batpred I didn't write the Seplos BMS software, I a...
By Bash , 1 hour ago
-
RE: Testing new controls/monitoring for Midea Clone ASHP
@tasos and @cathoderay thanks. I have some history grap...
By benson , 2 hours ago
-

I am having my existing heat pump changed to a Vaillant...
By trebor12345 , 3 hours ago
-
Our Experience installing a heat pump into a Grade 2 Listed stone house
First want to thank everybody who has contributed to th...
By Travellingwave , 3 hours ago
-
RE: Setback savings - fact or fiction?
@cathoderay The input power is largely determined by...
By RobS , 4 hours ago
-

RE: Solis inverters S6-EH1P: pros and cons and battery options
Just to wrap this up here for future readers: The S...
By Batpred , 6 hours ago
-
RE: Struggling to get CoP above 3 with 6 kw Ecodan ASHP
Welcome to the forums.I assume that you're getting the ...
By Sheriff Fatman , 6 hours ago
-
RE: Say hello and introduce yourself
@editor @kev1964-irl This discussion might be best had ...
By GC61 , 7 hours ago
-
@painter26 — as @jamespa says, it's for filling and re-...
By cathodeRay , 11 hours ago
-

RE: Oversized 10.5kW Grant Aerona Heat Pump on Microbore Pipes and Undersized Rads
@uknick TBH if I were taking the floor up ...
By JamesPa , 22 hours ago
-

RE: Getting ready for export with a BESS
I would have not got it if it was that tight
By Batpred , 24 hours ago
-
RE: Need help maximising COP of 3.5kW Valiant Aerotherm heat pump
@judith thanks Judith. Confirmation appreciated. The ...
By DavidB , 1 day ago
-

RE: Recommended home battery inverters + regulatory matters - help requested
That makes sense. I thought better to comment in this t...
By Batpred , 1 day ago
-
Bosch CS5800i 7kW replacing Greenstar Junior 28i
My heat pump journey began a couple of years ago when I...
By Slartibartfast , 1 day ago
-

RE: How to control DHW with Honeywell EvoHome on Trianco ActiveAir 5 kW ASHP
The last photo is defrost for sure (or cooling, but pre...
By JamesPa , 1 day ago
-

RE: Plug and play solar. Thoughts?
Essentially, this just needed legislation. In Germany t...
By Batpred , 1 day ago
-
RE: A Smarter Smart Controller from Homely?
@toodles Intentional opening of any warranty “can of wo...
By Papahuhu , 1 day ago
-
RE: Safety update; RCBOs supplying inverters or storage batteries
Thanks @transparent Thankyou for your advic...
By Bash , 1 day ago
-
RE: Air source heat pump roll call – what heat pump brand and model do you have?
Forum Handle: Odd_LionManufacturer: SamsungModel: Samsu...
By Odd_Lion , 1 day ago
-
RE: Configuring third party dongle for Ecodan local control
Well, it was mentioned before in the early pos...
By F1p , 2 days ago
-

RE: DIY solar upgrade - Considering adding more panels
I know this is a bit old, but it made me wonder what co...
By Batpred , 2 days ago